JW
JW’s acquisition of Vietnamese Euvipharm zeroes in on the global pharmerging market

JW Group, a leading player with differentiated technological capability in the Korean pharmaceutical market, has set out to penetrate the global pharmerging market by acquiring a 100 equity of a Vietnamese pharmaceutical company Euvipharm. With the acquisition, it has built a foothold to advance into the global market. Here’s a more story about JW always being a trailblazer in the Korean pharmaceutical industry from the development of first-in-class drugs to the export of a third-generation TPN and pharmaceutical raw materials.
As part of the pharmerging markets, Vietnam is an emerging blue ocean.
The word “pharmerging” is a recently-coined slang combining “pharmacy” and “emerging” to refer to newly emerging pharmaceutical markets of developing countries with higher growth potential but smaller clinical costs than those of developed countries. China, Vietnam, Brazil, and Mexico are the major countries.
Even until the mid-2000s, the US and Europe together dominated about 70% of the global pharmaceutical market, and the portion of these pharmerging markets was merely 14 % or so. Since 2011, however, the portion has grown rapidly to reach about 20~30% now.
These pharmerging markets have expanded about 10.3% annually over the past 5 years, twice as fast as developed ones. Fueled by this growth rate, the size of these new markets is expected to swell from 270 billion dollars in 2017 to hit about 375 billion dollars.
The market of Vietnam, in particular, a country to which many Koreans now feel more friendly with the success story of Park Hang-seo, the South Korean head coach of the country’s men's national football team, recorded about 4.7 billion dollars (5689.4 billion won) in size in 2016 and has been marking 11% of an annual growth rate since then
New opportunities may lie in overseas markets! Korean pharmaceutical companies are eyeing the pharmerging markets
To overcome the limits of the domestic market, Korean pharmaceutical companies are seeking to directly enter markets abroad because the export of domestically produced products has its limits in market expansion due to the grading of pharmaceutical products, price competitiveness, and other issues.
The direct entry into overseas markets was limited to building a local subsidiary or launching a promotional campaign until recently, but now an increasing number of Korean companies are diversifying their strategies to participate in company operation or securing manufacturing infrastructure by acquiring part of the company equity or building a factory overseas.
A first-mover JW ventures into Vietnam!
JW Group signed a deal to acquire a 100% share of Euvipharm, a pharmaceutical raw material and finished goods producer based in Long An Province, Vietnam on September 4, 2019.
The group had performed thorough market research to confirm its competitiveness in the emerging pharmaceutical markets and selected Vietnam, a country demonstrating a noticeable growth rate and strong potential to grow even further, as the group’s outpost to break into the pharmerging markets.
And then, in April 2018, it participated in the Global M&A Support Project led by the Korea Trade-Investment Promotion Agency (KOTRA) to step up its effort to identify an investment destination. The result would be a win-win business opportunity for both JW and a prospective investee, so the M&A team could smoothly collaborate with the KOTRA office in Ho Chi Minh City. Negotiations with Euvipharm, the selected finalist for acquisition, also went well on a positive note.
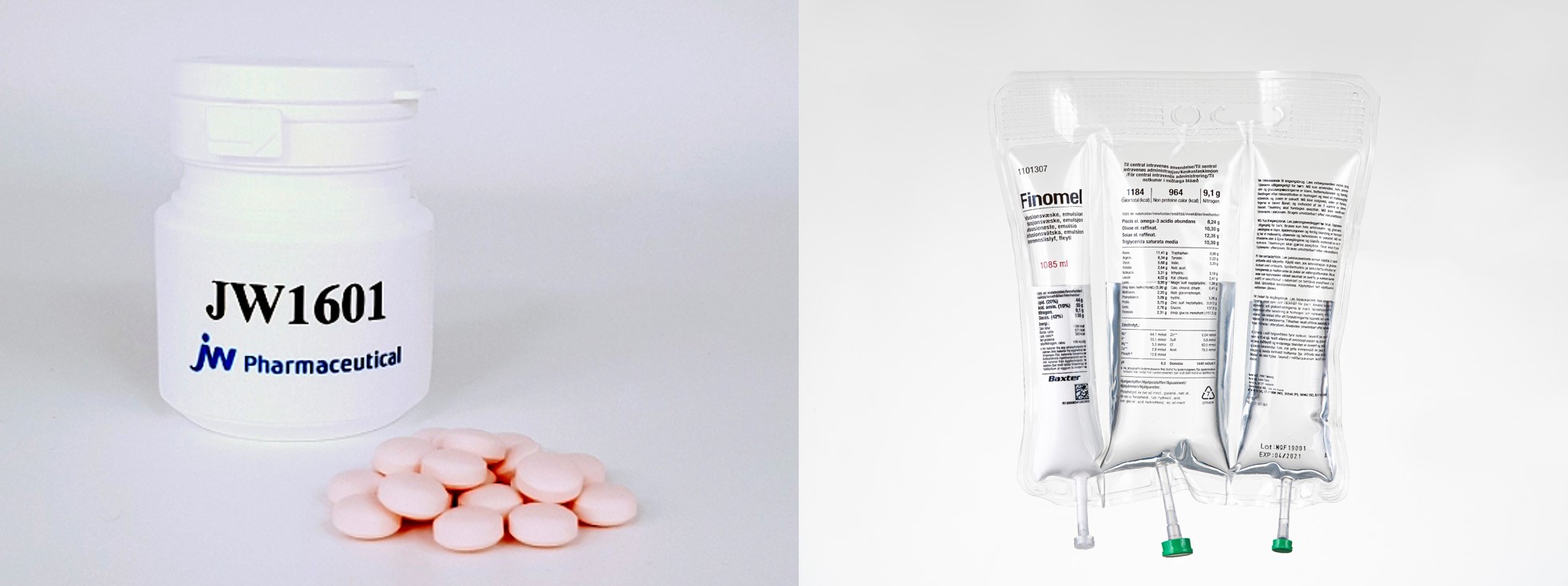
The acquisition of a Vietnamese pharmaceutical company manifests JW’s another strategy to expand into the pharmerging markets following its focus on first-in-class drugs and the PN market. JW licensed out JW1601, an atopy treatment, to Leo Pharmaceutical, and launched the TPN Finomel in the European markets as the first Asian pharmaceutical company.
Going forward, JW Group will transfer its original knowhow to Euvipharm in stages and implement its vision by seeking certification under the PIC/S (Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme) and launching a CMO Contract Manufacturing Organization) business.
“All eyes in the global market will be on potential synergy effects that JW’s technology and the local infrastructure in Vietnam will create,” said the CEO Sung-nam Cha. “From Vietnam as our outpost, we will accelerate our market expansion not only to the ASEAN markets but also more advanced ones such as Europe or America.”
* About Euvipharm
Euvipharm is a robust Vietnamese pharmaceutical company founded in 2005. It was taken over by Valeant Pharmaceuticals (currently Bausch Health Companies), the largest pharmaceutical company in Canada, in 2013 and operated by the company since then. Its factory is WHO-GMP certified and has one of the most modernized manufacturing lines in Vietnam. On its massive premises, a 35,000m² in total area, it can churn out 1,937 million units of products a year.
Please make sure to indicate the source (JW Group Newsroom) when using the content.
Popular posts